Leveraging SeerPharma’s vast and extensive experience working in the industries to which we consult, you can benefit from our ability to quickly understand your areas of concern and then provide pragmatic and practical advice. With a thorough working knowledge of all relevant industry regulations and guidance, we help clients like you implement effective and compliant business practices to improve overall performance.
Click here to learn more about how we can help you virtually with:
- General Quality Assurance and GMP compliance concerns
- Computer System Validation (including setting up a CSVMP and Infrastructure Qualification framework)
- Desktop auditing
- Reviewing Facility Designs for GMP Compliance
- Reviewing Supplier Agreements
- Virtual GMP training
Contact us for advice and assistance in of the following or similar areas:
Codes and regulations such as:
- Pharmaceutical Inspection Co-operation Scheme Guide to Good Manufacturing Practice for Medicinal Products (PIC/S Guide to GMP) PE 009
- European Union (EU) GMP Guidelines (EudraLex - Volume 4)
- Code of Federal Regulations (CFR) Title 21, Parts 11, 210, 211
- Australian Code of Good Manufacturing Practice for Human Blood, Blood Components, Human tissues and Human cellular therapy products
- Australian Code of Good Manufacturing Practice for Veterinary Chemical Products
- Australian Code of Good Wholesaling Practice for Medicines in Schedules 2, 3, 4 and 8
Regulatory bodies such as:
- Therapeutic Goods Administration (TGA) in Australia
- Medsafe in New Zealand
- Health Sciences Authority (HSA) in Singapore
- Food and Drug Administration (FDA) in the United States
- Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom
Quality standards and guidelines such as:
- ISO 9001: Quality management systems -- Requirements
- ISO 13485: Medical devices -- Quality management systems -- Requirements for regulatory purposes
- ICH Quality Guidelines including but not limited to:
- ICH Q9 Quality Risk Management
- ICH Q10 Pharmaceutical Quality System
Products (including software)
- Pharmaceuticals (all dosage forms, sterile and non-sterile)
- Medical devices, Software as a Medical Device (SaMD), in-vitro diagnostic devices
- Biologicals
- Cellular, blood and tissue therapies
- Software products
- Quality Management Systems (QMS)
- Learning Management System (LMS)
- Laboratory Information Management Systems (LIMS)
- Enterprise Resource Planning (ERP) or Material Resource Planning (MRP)
- Manufacturing Execution Systems (MES)
Quality and GMP Areas
- Good Manufacturing Practices (GMP)
- Good Practices such as Clinical, Laboratory and Distribution/Wholesaling (GxP)
- Facility design and licensing (including FDA and TGA)
- Validation Master Plan (VMP) and execution
- Quality Assurance (QA) and Quality Management Systems (QMS)
- Data management and data integrity
- Internal and external audits and inspections
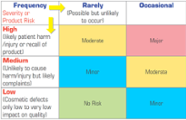
- Risk assessment and risk management
- Vendor assurance and supply chain management
- Computer Systems Validation (CSV)
- IT Project Management
- Equipment qualification
- Process validation
- Analytical method validation
- Contamination control
- Product Quality Reviews (PQR)
- Release for supply
- Conformity assessment
- Continuous improvement
- Regulatory Affairs (RA)