Company Background
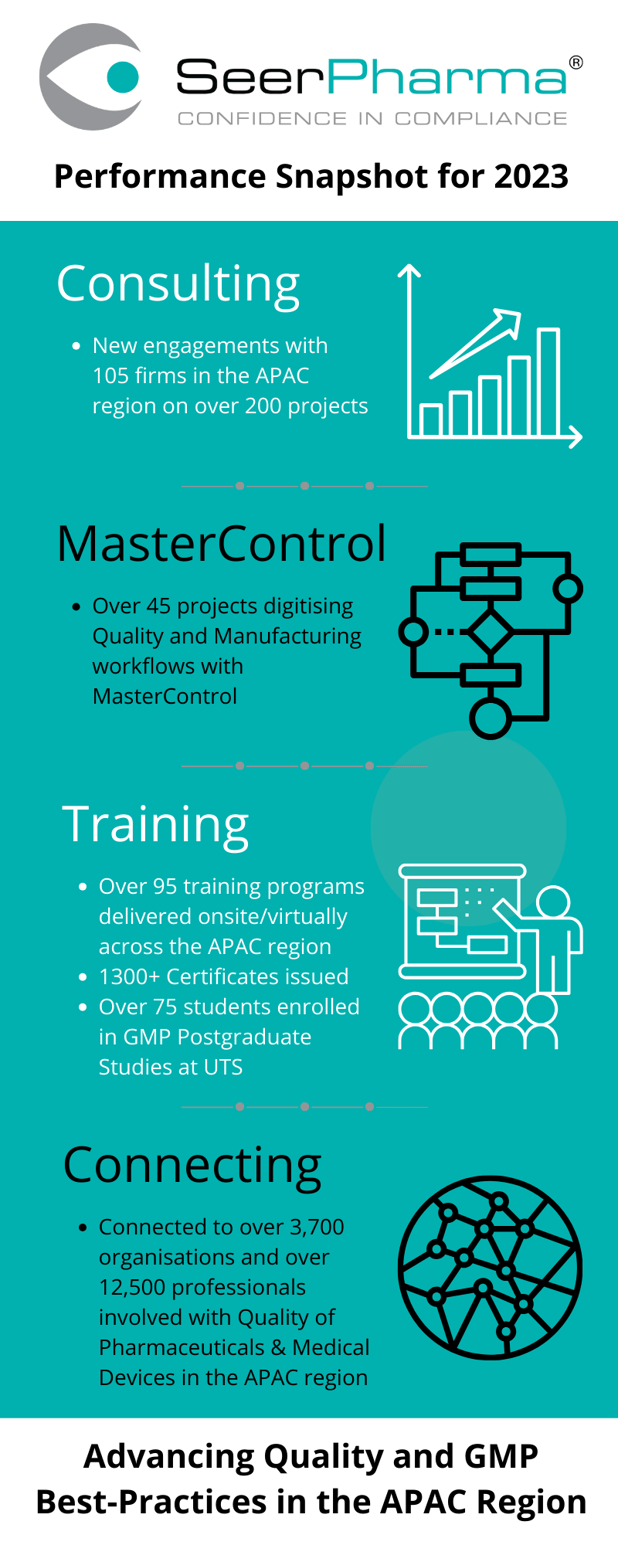
SeerPharma is a team of consultants that provide best-practice advice, training, software solutions from MasterControl, and contract labour resources to pharmaceutical and medical device companies in the Asia-Pacific (APAC) region on matters related to Quality and GMP compliance. Our clients are leading organisations, involved with, and/or conducting step(s) of manufacture of pharmaceutical and medical devices.
We have been serving our clients in the Asia-Pacific region for over 35 years from Melbourne, Sydney, and Singapore, and are also able to assist in Europe through our affiliate in the UK.
We work with organisations looking to ensure that their facilities, equipment, computer/IT systems, quality systems, and processes are compliant with FDA, PIC/S, TGA, WHO, ISO, ICH, and other regulations and standards that apply to their product(s). Clients turn to us for assistance when having to adopt changes to these regulations/standards. However, most enquiries to SeerPharma, are driven by when clients are driving change in their operations, typically for commercial reasons, which then impacts their state of GMP compliance.
We are routinely engaged to help our clients prepare for and/or respond to GMP inspections from regulators such as the TGA, US FDA, and EMA. We regularly inspect organisations on behalf of the Australian Government (APVMA) as well as Pharmaceutical and Medical Device organisations relying on suppliers in the Asia-Pacific region.
Our consultants actively train companies on Quality/GMP compliance from Research and Development through to Manufacturing, Logistics, Warehousing, and Post Market Surveillance. Our passion for training has led us to develop and deliver an internationally recognised Postgraduate GMP program at the University of Technology Sydney (UTS) unique to the Asia-Pacific region.
Our Software Solutions team routinely implements electronic Quality Management Systems, and paperless manufacturing solutions from market-leading software provider MasterControl to help improve process efficiencies, and help our clients better demonstrate GMP compliance.
Companies also turn to SeerPharma to provide short-term Quality Assurance contractors to help with projects that do not warrant hiring a full or part-time employee.
Through everything we do, we aim to "Advance Quality and GMP Best Practices in the APAC region" for the Pharmaceutical and Medical Device Industries.
Contact us to explore how you can leverage our skills and services to support your business needs.
Learn more about our Leadership Team